leak test cycle autoclave|steam penetration test autoclave : tv shopping How to Run a Bowie-Dick Test Recommended use of a Bowie-Dick Test is outlined in the Association for the Advancement of Medical Instrumentation's (AAMI) guidance ANSI/AAMI ST79 Comprehensive Guide to steam sterilization and sterility assurance in health care facilities.Bowie-Dick Tests are used to qualify sterilizer performance when first installed, when . $2,250.00
{plog:ftitle_list}
The Little Sister SES 3020B has been designed to meet the needs of the healthcare professional, building on Eschmann’s unrivalled reputation for quality and performance gained from over 60 years of autoclave design and .
What is a Steam Sterilizer (Autoclave) Leak Test? A vacuum leak test is used to verify the integrity of the sterilizer pressure vessel and its plumbing. Steam sterilizer operator manuals . The Vacuum Leak Test programmed on your autoclave only measures the integrity of the sealed pressure vessel and associated piping to ensure air is not admitted to the .
abcam p24 elisa kit
The vacuum test comprises several phases which are defined in the program sequence of the autoclave:. Evacuation phase: the sterilization chamber is evacuated until the required vacuum pressure is reached. Equalization time: This is followed by an equalization time of 5 minutes to ensure that the pressure in the chamber is stable.. Measuring time: The measuring time is 10 . Acceptance criteria: For the thermometric test, it is essential to achieve 121°C for each thermocouple for a predefined time period of 15 minutes. the difference between the temperature and thermocouple should not be more than 3°C that are all the thermocouples must have a temperature between 121°C to 124°C during the entire sterilization . How to Run a Bowie-Dick Test Recommended use of a Bowie-Dick Test is outlined in the Association for the Advancement of Medical Instrumentation's (AAMI) guidance ANSI/AAMI ST79 Comprehensive Guide to steam sterilization and sterility assurance in health care facilities.Bowie-Dick Tests are used to qualify sterilizer performance when first installed, when .
Anatomy of a Steam Sterilization Cycle. Steam sterilization cycles can be divided into three distinct phases; conditioning, exposure and drying. . The Bowie-Dick test is conducted daily to ensure that the vacuum system is adequately removing air from the chamber and should be used in conjunction with a weekly leak test to determine if there .
abcam pge2 elisa kit
The test pack is placed into an empty chamber, and a pre-vacuum cycle is then activated. If the test fails, it indicates that the autoclave has leak problems. Bowie and Dick test indicators As we can see in the picture above: •Unexposed: Initial color stays the same . A typical Vacuum Leak Test Cycle will consist of three alternating vacuum . F 0 Cycle: Sterilization begins when temperature reaches a minimum of 212F and is completed when the F0 set-point is achieved. F0 is adjustable. Heat-sensitive media and liquids: Validation, Bowie-Dick Test: Daily air removal test, typically for healthcare applications: Validating the sterilizer. Validation, Vacuum Leak Test Performance Qualification Protocol for Steam Sterilizer (Autoclave) and Procedure for Vacuum Leak Test, Steam Quality Test, Bowie-Dick Test, Heat Distribution Test, Hest Penetration Test and F0 Calculation. . The printout taken during the Bowie-Dick test cycle & the Bowie-Dick test indicator should be preserved as per the Annexure-3 Attachment-3.and air removal test sheets from the Air Leak Cycles are provided in Figure 4. Table 4: Bowie-Dick Test Results – Air Leak Cycles Test Type Lot Number Cycle 1 Result Cycle 2 Result 3M Comply 1233LF 2015-10AD FAIL FAIL Steris DART M13113 FAIL FAIL PCD Test Results – Air Leak Cycles The results of the indicators contained in the PCDs tested
abcam rhk 324-01r myeloperoxidase mpo elisa kit 96w
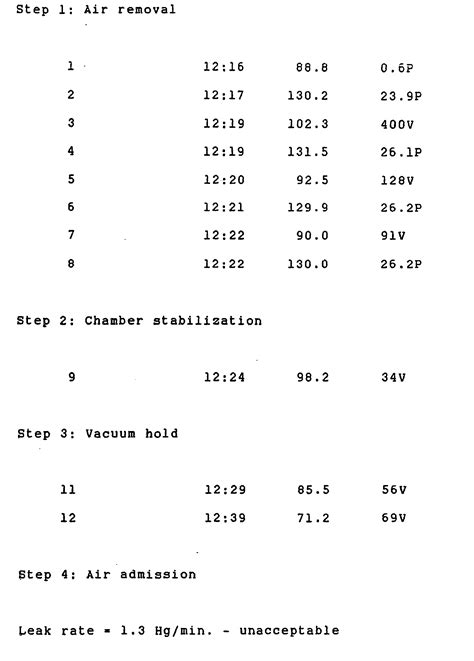
Three vacuum/pressure pulses are typically used in a vacuum leak test cycle, which is followed by a 15-minute dwell time at deep vacuum. After the cycle is over, the autoclave’s control panel will show the leak rate in units like psia/min, kPa/min, mbar/min, or mmHg/min.
Begin the Bowie-Dick test cycle. While parameters for these cycles aren’t specifically defined in the ANSI/AAMI ST79, general guidance recommends three-and-a-half to four minutes of exposure time. However, many modern autoclaves have pre-programmed cycles that are specifically designed for running Bowie-Dick tests. Examine the test sheet and .Related: Autoclave Basic Components, Leak Test used in Autoclave, Autoclave Types, biological indicator for autoclave. Important Parameters of an Autoclave. The following factors affect how an autoclave operates and needs to be maintained: Autoclave Temperature and Pressure What is leak test in autoclave? Vacuum leak tests are used to determine the airtight integrity of chambers and piping systems in pre-vacuum autoclave. The test exposes the pipes and components of the Steam Steriliser autoclave to vacuum conditions and measures the extent of vacuum depth loss over a given period of time. Vacuum leak testing is .N o te s : AtypicalLeakTestcyclewilloperateasfollows: StageON E willbetheinitialpull-down. Onceatthepre-setvalue(A),thevacuumpumpwill STOP StageTWO isthefirsttimer .
How to Validate an Autoclave 8 Cycle development is performed to provide you with a sterilization process (i.e. cycle type, sterilization temperature, sterilization time, etc.) that can be validated. . tion time (this is referred to as the “half-cycle” method). Sterility testing is conirmed with the use of biological indicators (BIs). The .
An autoclave is a machine that uses steam under pressure to kill harmful bacteria, viruses, fungi, and spores on items that are placed inside a pressure vessel. The items are heated to an appropriate sterilization temperature for a given amount of time.The Vacuum Leak Test is used to determine the air-tight integrity of a pre-vacuum autoclave’s chamber. Vacuum leak .
example with a bioburden of 10 CFU, the ideal cycle at 121.1°C can be shortened from a 30-minute overkill cycle to a 17.5-minute cycle (7 log x 2.5 min./log). Figure 3 shows the sterilization time required at 121.1°C for an ideal cycle to achieve a SAL of 10-6 at varying levels of bioburden (D-value = 2.5 min.). Vacuum and Non-Vacuum Cyclesthe autoclave user For tightness testing purposes, the chamber is pressurized by compressed air up to 2 to 3 bar abs A long stabilization time (up to 30 minutes) is to be foreseen to eliminate the . Verify the efficacy of the air removal phases of the tested cycle Conducting an air leak test will identify chamber leaks. A steam-quality test .
vacuum leak test acceptance criteria
sterilizer leak test parameters
Vacuum leak test Priorclave autoclaves include a fully automated vacuum Leak Test Cycle program. Running this program automatically checks the integrity of the chamber and interconnecting pipe-work. During a Leak Test Cycle, the autoclave evacuates the chamber to your established vacuum set-point, then holds for five minutes before checking the pressure again.
The different tests are follows for qualification of autoclave are: – Vacuum leak test Bowie-dick test Heat distribution study. . Start the vacuum leak rate test cycle and observe the pressure in the pressure gauge of steam sterilizer and cycle allow the pressure to drop down. Machine will close all the valves connected to the chamber and .
steam penetration test autoclave
Both documents also address qualification testing under section 10.6.4, “Sterilization process failures.” Different types of qualification methods are used, depending on the type of sterilizer and cycle. The test-ing always uses a BI and it may also include the use of a Bowie-Dick test, which is a sensitive and rapid meansVacuum Leak Test (Ref. SOP VAL-175) Form: 805 Issue date: Version: 1 Page: 1 of 2 . 1. Procedure. A Vacuum Leak Test is performed to test the integrity of the autoclave. This form must be printed for each consecutive test. 1.1 Vacuum Leak Test Performance . 1.1.1 Synchronise the time of the Graphic Recorder (Eurotherm Chessell 6000Autoclave‘s vacuum leak test, which only assesses the integrity of the sealed pressure vessel and associated piping, confirms that air is not being sucked (Drawn) into the sterilizer during vacuum drawdowns. . ANSI/AAMI ST79 does not deliver exact guidance on test cycle parameters. However, they specify that the programmed exposure time .
AUTOCLAVE VALIDATION PROTOCOL (Ref. SOP VAL-175) Form 780 Issue date: Version: 1 Page: 1 of 2 . 1. Procedure . Cycle Review Form-765) must be completed and attached. Sign/Date . . 1.3.2 Conduct a Vacuum Leak Test using Form-805 (Vacuum Leak Test). Record all test instances
Many dynamic air removal steam sterilizers have a leak test built into the machine. This test measures the leakage of air into the chamber. . A specified cycle is run, and the test sheet must show a uniform color change. Results that are not uniform are considered a failure. . Lewis PE, and Raymond G. Practical Guide to Autoclave Validation. The presence of air in an autoclave sterilisation cycle adversely affects steam penetration and contact with materials being sterilised. It is important to routinely perform an air removal verification test in the autoclave to demonstrate that entrapped air is removed and thus cannot impede the steam sterilisation process. . An integral, leak .
8.1 Vacuum leak test Procedure 8.1.1 Put the flexible probes inside the chamber through the validation port provided for the validation cycles prior to start of Vacuum leak test. 8.1.2 Ensure that Autoclave is empty and chamber is at ambient temperature. 8.1.3 Ensure compressed air is ‘ON’ at required pressure of 6 bar.

abcam serotonin elisa kit
abcam simplestep elisa kit
Browse a full range of Sterilizer and Autoclave Accessories products from leading suppliers. Shop now at Fisher Scientific for all of your scientific needs.Combine compact design with high performance, reliability and safety. Intelligent design for .
leak test cycle autoclave|steam penetration test autoclave